electronic logbook software pharmaceutical|compact electronic logbook system : iloilo Electronic logbooks effectively manage the statuses and activities of manufacturing . The Legend of Zelda: Breath of the Wild [Note 1] (ou simplement Breath of the Wild, parfois abrégé BotW) est un jeu d'action-aventure développé par la division Nintendo EPD, assisté par Monolith Soft, et publié par Nintendo le 3 mars 2017 sur Nintendo Switch lors du lancement de la console, ainsi que sur Wii U dont il est le dernier jeu produit par Nintendo.
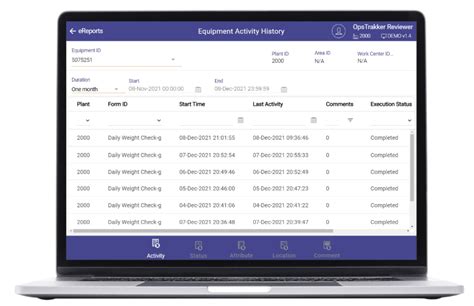
electronic logbook software pharmaceutical,OpsTrakker offers a digital logbook solution that eliminates paper use-logs and logbooks in GMP manufacturing facilities, enables real-time data capture and equipment status .
Electronic logbooks effectively manage the statuses and activities of manufacturing .
Knowledge Base. Electronic Equipment Use-Logs and Logbook Management. .The Vimachem Digital Forms and Logbooks module is a cloud-based forms & logbooks design and bookkeeping solution that digitizes within minutes any paper-based logbook and form found in pharma and .
Learn how logbook software can help pharmaceutical manufacturers comply with regulatory standards, improve data integrity, and enhance efficiency. Compare the .Electronic Logbook Software. Digitize Your Operations with AmpleLogic eLogbook. Schedule a Free Demo. Superior Data Management with AmpleLogic eLOGS. .
CalibereLog works as e-register to store details at warehouse, QC, QA, and other departments. The e-logbook can be issued, stored, and retrieved during audit trails .electronic logbook software pharmaceuticalElectronic Logbook For Pharma And Manufacturing Companies. Key Features. Organized And Collaborative. Simple use & Rich text editor. Collaborative environment & workflow. Can be integrated to other .SAP, ServiceNow and LabWare are commonly requested. It can also integrate with the full ValGenesis VLMS. Explore ValGenesis e-Logbook, the web-based electronic logbook .Enforces GDP compliance. Logbook entries can be reviewed in real time from any place. Eliminates all physical logbook document management, storage, retrieval time loss and .CalibereLog works as e-register to store details at warehouse, QC, QA, and other departments. The e-logbook can be issued, stored, and retrieved during audit trails inexpensively. Send alerts, maintain punches & dies issuance logs conveniently with CalibereLog. Inspection and discard rules of punches can be defined and restrictions on .
Yes. ValGenesis e-Logbook can integrate with applications such as MES, QMS, ERP and more to collect related log records. SAP, ServiceNow and LabWare are commonly requested. It can also integrate with the full ValGenesis VLMS. Explore ValGenesis e-Logbook, the web-based electronic logbook software built for pharmaceutical, . Electronic logbooks, eLogBooks, or e-logs in the pharmaceutical industry aren’t exactly new. Many manufacturers have been using software for years to track equipment, production, and manufacturing of raw materials and finished goods. eLogBooks reduce paper use by eliminating paper documents, improving visibility into production .
compact electronic logbook systemRecord and manage both events and instructions. With its simpified data-entry process requiring minimal keyboard use, eLogBook is ideal for recording and then managing plants events and issuing instructions. Track progress. Track the progress of 'open' events and monitor all associated actions. Create audit trails.
Superior Data Management with AmpleLogic eLOGS. AmpleLogic eLogbook revolutionizes data management in regulated industries such as Life Sciences, Food & Beverages, Cosmetics, Gene Therapy, Medical Devices, and more. Our web-based software replaces manual logbooks, guaranteeing compliance, efficiency, and transparency in your .
7. Elogbook Software: Elogbook software is used in the pharmaceutical industry to replace traditional paper-based logbooks and recordkeeping systems. It provides a digital platform for researchers, scientists, and engineers to record their activities, observations, and experiments electronically. 8.DiceNa. Sep 3, 2013. #1. We are currently using paper logbooks in our 21 CFR part 211 environment. The logs specifically deal with equipment usage, cleaning and maintenance as called out by CFR 211.182. The paper books we currently use have some serious limitations. I have been searching the internet for weeks and I have been unable to . When assessing different electronic solutions, selecting one compliant with 21 CFR Part 11 is essential. OpsTrakker was built with GMP in mind to deliver the requirements necessary for user identification, authorization, traceability, and reliability. OpsTrakker equipment management, forms, and documentation solutions can help .AmpleLogic Audit Management Software is a comprehensive solution designed to streamline and enhance the audit processes for organizations operating in regulated industries such as pharmaceuticals, biotech, medical devices, and others. This software offers a range of features and benefits tailored to meet the specific needs and .

Superior Data Management with AmpleLogic eLOGS. AmpleLogic eLogbook revolutionizes data management in regulated industries such as Life Sciences, Food & Beverages, Cosmetics, Gene Therapy, Medical Devices, and more. Our web-based software replaces manual logbooks, guaranteeing compliance, efficiency, and transparency in your .AmpleLogic BRIMS: Streamlining Batch Record Management. AmpleLogic BRIMS is a robust software solution designed to streamline and automate batch record management processes in pharmaceutical, biotech, and life sciences industries. With compliance to regulatory standards such as 21 CFR Part 11, cGMP, GMP, MHRA, EU Annex 11, and . Best electronic logbook software tools. Jotform: Best for customization. eLogger: Best for manufacturers. Everlance: Best for drivers of any distance. eRIS-LOG: Best for sharing images and videos. .In the pharmaceutical industry, where adherence to stringent regulatory standards is paramount, Quickflow offers a comprehensive Electronic Logbook (eLogbook) solution that revolutionizes data recording and management. . Quickflow Electronic Logbook Software ensures compliance with FDA regulations, notably 21 CFR Part 11, and aligns .Product Description. How are these determined? SciNote is a cloud-based electronic lab notebook (ELN) with built-in inventory, compliance, & team management tools. It is trusted by the FDA, USDA, European Commission, and 100K+ scientists in ov.
Electronic Logbook Solution abled to create master data for equipment, instruments and functional locations. Entries made in the eLog are real-time and ensures review, verification, and approvals on time. Logbook data is stored electronically in a centralized repository and is accessible at all times for further analysis or audits.
Both GMP and 21 CFR Part 11 compliant, OpsTrakker provides for electronic equipment status management and digital record keeping to achieve the following business benefits: Facilitate Compliance. Improve Operations. Unlock Manufacturing Data. Available for iOS, Android, and supported browsers, advance your digitalization strategy with OpsTrakker.

Maintaining an equipment logbook is a mandatory requirements as per FDA 21 CFR 211.182 Equipment cleaning and use log requirements. Equipment Log Management (ELOG) is one of the essential elements of a quality management system. . About the company Pharma Software Solutions is a software company aimed to develop the .electronic logbook software pharmaceutical compact electronic logbook systemMaintaining an equipment logbook is a mandatory requirements as per FDA 21 CFR 211.182 Equipment cleaning and use log requirements. Equipment Log Management (ELOG) is one of the essential elements of a quality management system. . About the company Pharma Software Solutions is a software company aimed to develop the .
electronic logbook software pharmaceutical|compact electronic logbook system
PH0 · what is valgenesis
PH1 · logbook management software
PH2 · handheld electronic logbook
PH3 · gmp electronic logbook
PH4 · electronic maintenance logbook software
PH5 · electronic logbook systems
PH6 · electronic log software
PH7 · compact electronic logbook system
PH8 · Iba pa